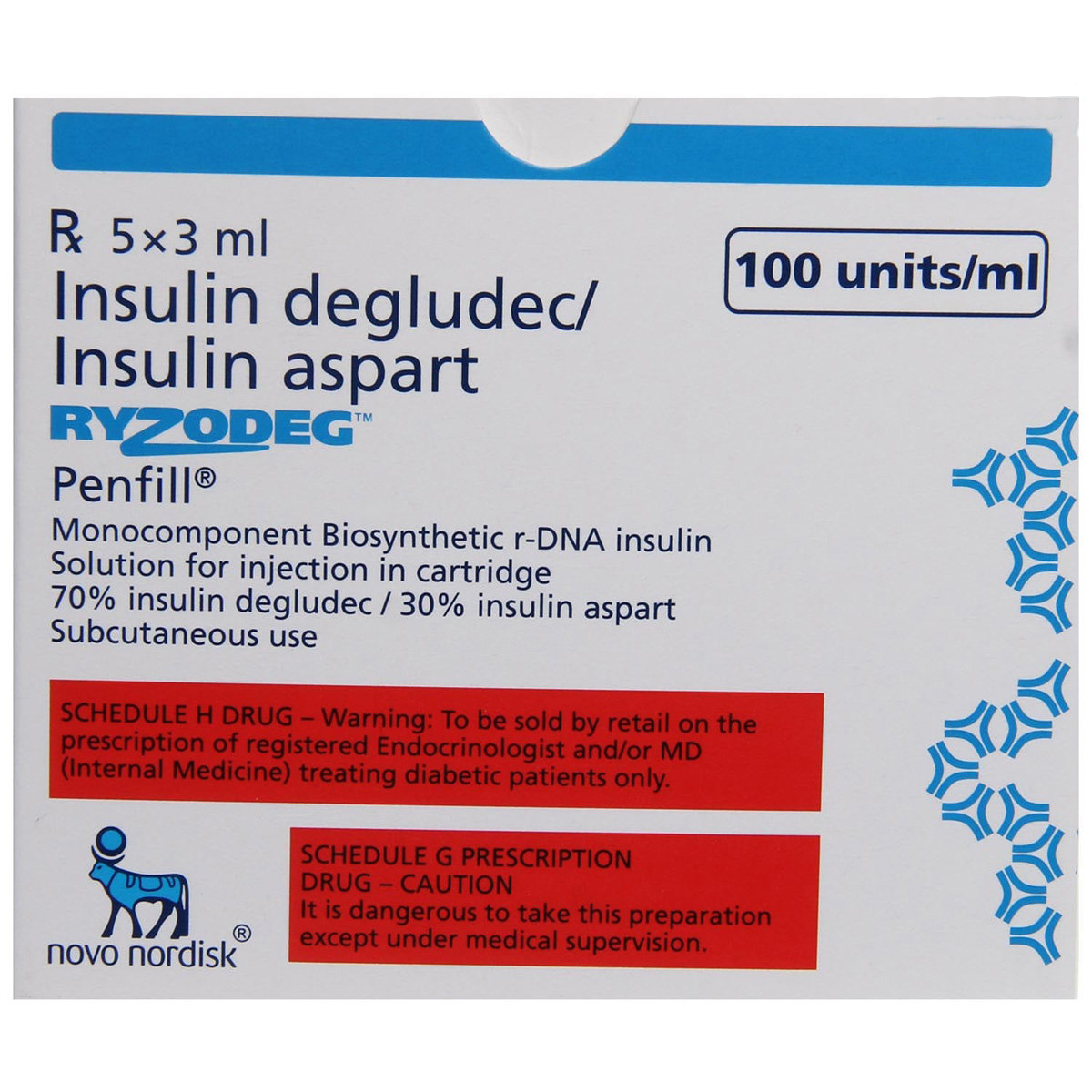
Ryzodeg 100IU/ml Flextouch Pen 3 ml
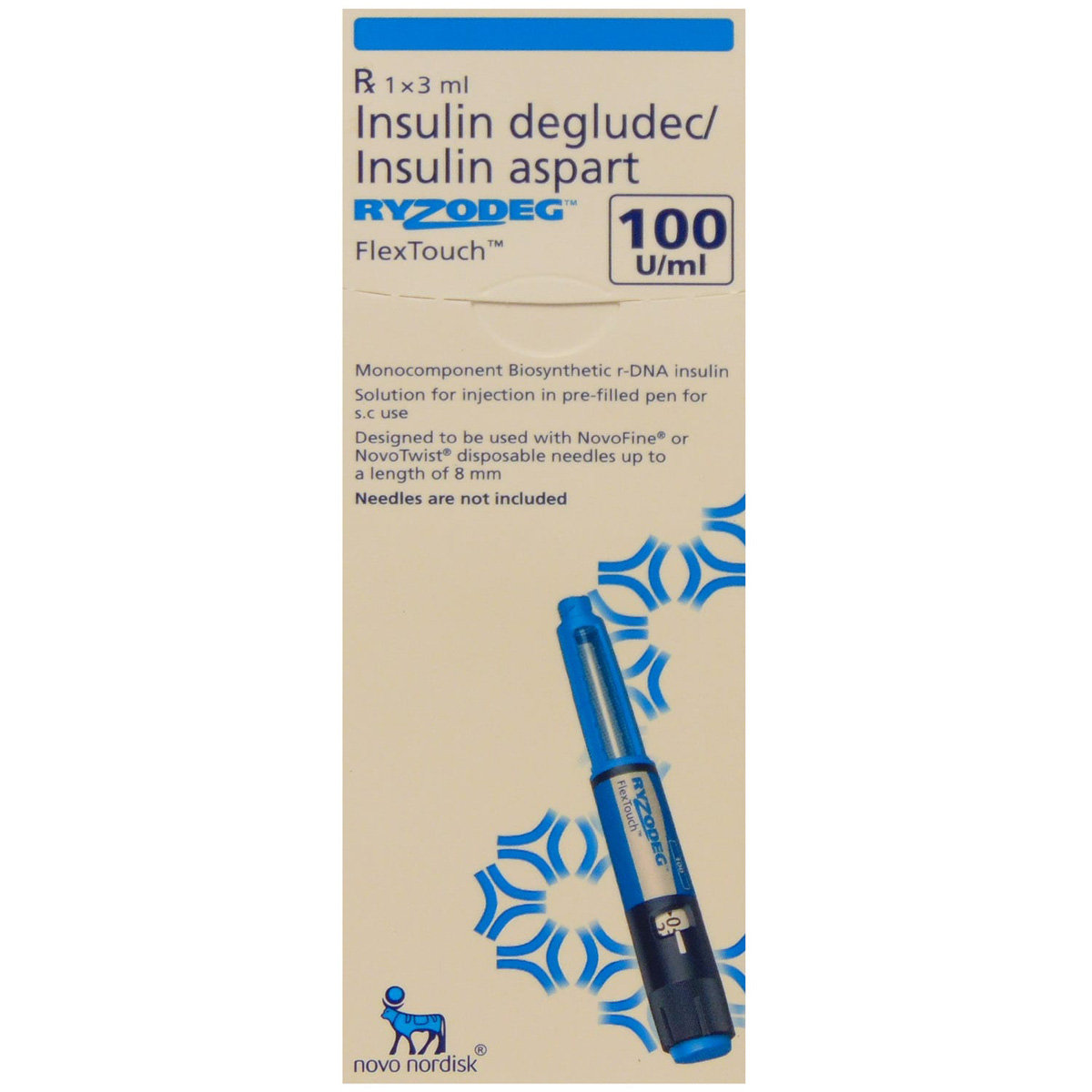

MRP ₹1595
(Inclusive of all Taxes)
₹239.3 Cashback (15%)
know your delivery time
Provide Delivery Location
Composition :
Manufacturer/Marketer :
Consume Type :
Expires on or after :
Return Policy :

Secure Payment

Trusted by 8 Crore Indians

Genuine Products
Therapeutic Class
Country of origin
Manufacturer/Marketer address
Disclaimer
Alcohol
Safe if prescribed
You are recommended not to consume alcohol along with Ryzodeg 100IU/ml Flextouch Pen 3 ml to avoid unpleasant side-effects. Alcohol may either decrease or increase the blood sugar level which can be fatal.
Pregnancy
Consult your doctor
Ryzodeg 100IU/ml Flextouch Pen 3 ml can be used during pregnancy. Your insulin dose may need to be changed during pregnancy and after delivery.
Breast Feeding
Consult your doctor
Ryzodeg 100IU/ml Flextouch Pen 3 ml can be given safely to nursing mothers but only under the supervision of a doctor.
Driving
Safe if prescribed
Drive with caution, Ryzodeg 100IU/ml Flextouch Pen 3 ml usually causes drowsiness and affects driving ability. Your ability to concentrate and react may be reduced if you have hypoglycemia (low blood sugar).
Liver
Consult your doctor
Ryzodeg 100IU/ml Flextouch Pen 3 ml to be taken with caution, especially if you have a history of liver diseases/conditions. The dose may have to be adjusted by your doctor.
Kidney
Consult your doctor
Ryzodeg 100IU/ml Flextouch Pen 3 ml to be taken with caution, especially if you have a history of kidney diseases/conditions. The dose may have to be adjusted by your doctor.
Children
Safe if prescribed
Ryzodeg 100IU/ml Flextouch Pen 3 ml can be given safely to children; dose has to be prescribed under the supervision of a doctor.
Product Substitutes
Keep Refrigerated. Do not freeze.Prepaid payment required.
About Ryzodeg 100IU/ml Flextouch Pen 3 ml
Ryzodeg 100IU/ml Flextouch Pen 3 ml belongs to the class of medications called ‘antidiabetics’ primarily used for the treatment of both type 1 and type 2 diabetes mellitus. It maintains blood sugar levels in adults and children. In Diabetes Mellitus Type 1, the body does not produce enough insulin to regulate blood sugar levels. On the other hand, in Diabetes Mellitus type 2, either the body stops producing enough insulin (a hormone that helps lower blood sugar levels), or there is resistance to insulin action. As a result, insulin is produced in large quantities, but it is not capable of operating on the body's organs.
Ryzodeg 100IU/ml Flextouch Pen 3 ml is a combination of two drugs, namely, Insulin Aspart and Insulin Degludec. Insulin aspart is a fast-acting or short-acting form of insulin that helps lower blood sugar levels after food intake. Insulin degludec is a long-acting form of insulin which begins working after 60 minutes and is a long-acting insulin preparation (lasts for more than 24 hours). Ryzodeg 100IU/ml Flextouch Pen 3 ml stimulates the recovery of sugar in muscle and fat cells and suppresses sugar production in the liver. Together, Ryzodeg 100IU/ml Flextouch Pen 3 ml confirm rapid and stable sugar control by helping reuptake of sugar in fat cells and muscle, and suppressing the creation of sugar in the liver.
Take Ryzodeg 100IU/ml Flextouch Pen 3 ml as prescribed by your doctor. You are advised to take Ryzodeg 100IU/ml Flextouch Pen 3 ml for as long as your doctor has prescribed it for you depending on your medical conditions. You may experience rash, injection site allergic reaction, weight gain, oedema (tissue swelling or fluid overload), lipodystrophy (skin thickening or pits at the injection site), hypoglycemia (low blood glucose level), itching. Most of these side effects of Ryzodeg 100IU/ml Flextouch Pen 3 ml do not require medical attention and gradually resolve over time. However, if the side effects are persistent, reach out to your doctor.
Try not to stop taking Ryzodeg 100IU/ml Flextouch Pen 3 ml of your own. Let your doctor know about this, as it may cause withdrawal symptoms. Do not take Ryzodeg 100IU/ml Flextouch Pen 3 ml if you have any low blood glucose levels, kidney, liver, or heart problems, or problems with alcohol or other prescription recreational drugs. Along with Ryzodeg 100IU/ml Flextouch Pen 3 ml, you should take a healthy diet, do regular exercise, and maintain weight as advised by your doctor. Ryzodeg 100IU/ml Flextouch Pen 3 ml is a cold chain medicine, and so it must be stored in the refrigerator between 2-8 degrees Celsius else its efficiency might get lost. Do not store in the freezer of the fridge.
Uses of Ryzodeg 100IU/ml Flextouch Pen 3 ml
Medicinal Benefits Mweb
Key Benefits
Ryzodeg 100IU/ml Flextouch Pen 3 ml belongs to the class of medications called ‘antidiabetics’ primarily used for the treatment of both type 1 and type 2 diabetes mellitus. Ryzodeg 100IU/ml Flextouch Pen 3 ml is a combination of two drugs, namely, Insulin Aspart and Insulin Degludec. Ryzodeg 100IU/ml Flextouch Pen 3 ml contains insulin aspart, which is a fast-acting form of insulin that helps lower blood sugar levels after food intake. Ryzodeg 100IU/ml Flextouch Pen 3 ml contains insulin degludec, which is a prolonged action form of insulin. It typically begins working after 60 minutes and is a long-acting insulin preparation (lasts for more than 24 hours). Together, they confirm rapid and stable sugar control by helping reuptake sugar in fat and muscle, and cells and suppressing the creation of sugar in the liver.
Directions for Use
Side Effects of Ryzodeg 100IU/ml Flextouch Pen 3 ml
- Rashes
- Injection site allergic reaction
- Weight gain
- Edema (swelling)
- Lipodystrophy (skin thickening or pits at the injection site)
- Hypoglycemia (low blood glucose level)
- Itching
Drug Warnings
Ryzodeg 100IU/ml Flextouch Pen 3 ml is for subcutaneous (under the skin) use only. However, in rare cases, it can be given via infusion intravenously under medical supervision. If you are changing the insulin brand or injecting your insulin by another method, it should be done under strict medical supervision. Cases of heart failure have been reported when pioglitazone was used with insulin, especially in patients at high risk of cardiac heart failure. The first hyperglycemia symptoms (high blood sugar level) may include excessive thirst, dry mouth, increased frequency of urination, nausea, vomiting, drowsiness, flushed dry skin, loss of appetite and acetone odour of the breath. You should closely monitor these symptoms. Symptoms like heart failure, weight gain and oedema (fluid deposition in tissue) should not be overruled. It is advisable not to consume alcohol as it may either increase or decrease your blood glucose level. Care should be taken while travelling across more than two time zones. Your doctor may adjust your insulin schedule. Ryzodeg 100IU/ml Flextouch Pen 3 ml may decrease the level of potassium, leading to a state of hypokalaemia that, if left untreated, may lead to respiratory paralysis, irregular heartbeat rhythm, coma, and even death. Do not take Ryzodeg 100IU/ml Flextouch Pen 3 ml if you have any low blood glucose levels, kidney, liver, or heart problems, or problems with alcohol or other prescription recreational drugs.
Drug-Drug Interactions
Drug-Drug Interactions
Login/Sign Up
Taking Ryzodeg 100IU/ml Flextouch Pen 3 ml with cinoxacin affects blood glucose levels, which may cause hyperglycemia (high blood glucose) and hypoglycemia (low blood glucose) less frequently.
How to manage the interaction:
There could be a possible interaction between Ryzodeg 100IU/ml Flextouch Pen 3 ml and Cinoxacin, it can be taken if prescribed by a doctor. However, consult the doctor immediately if you experience symptoms of hypoglycemia, which include headache, dizziness, drowsiness, nervousness, confusion, tremor, nausea, hunger, weakness, perspiration, palpitation, and rapid heartbeat, or symptoms of hyperglycemia which may include increased thirst, increased hunger, and increased urination. Do not stop using any medications without first talking to your doctor.
Taking Ryzodeg 100IU/ml Flextouch Pen 3 ml with trovafloxacin affects blood glucose levels, which may cause hyperglycemia (high blood glucose) and hypoglycemia less frequently.
How to manage the interaction:
There could be a possible interaction between Ryzodeg 100IU/ml Flextouch Pen 3 ml and trovafloxacin but can be taken if prescribed by a doctor. However, consult the doctor immediately if you experience hypoglycemia or hyperglycemia. Symptoms of hypoglycemia include headache, dizziness, drowsiness, nervousness, confusion, shaking, nausea, hunger, weakness, sweating, palpitation, and rapid heartbeat. Symptoms of hyperglycemia may include increased thirst, increased hunger, and increased urination. Do not stop using any medications without talking to a doctor.
Coadministration of levofloxacin and Ryzodeg 100IU/ml Flextouch Pen 3 ml can sometimes affect blood glucose levels - Both hyperglycemia (high blood glucose) and, less frequently, hypoglycemia (low blood glucose).
How to manage the interaction:
Although using levofloxacin and Ryzodeg 100IU/ml Flextouch Pen 3 ml at the same time may cause an interaction, they can be taken together if a doctor has prescribed it. Inform doctor if you develop hypoglycemia (low blood glucose) or hyperglycemia (high blood glucose), or if you have trouble controlling your blood glucose. Hypoglycemia symptoms include headache, dizziness, tiredness, anxiety, disorientation, a shaking sensation, nausea, hunger, weakness, sweating, palpitation, and a rapid pulse. Hyperglycemia symptoms include Increased thirst, increased hunger, and increased urination. Do not stop using any medications without talking to a doctor.
Taking Ryzodeg 100IU/ml Flextouch Pen 3 ml with nalidixic acid affects blood glucose levels, which may cause hyperglycemia (high blood glucose) and hypoglycemia (low blood glucose) less frequently.
How to manage the interaction:
There could be a possible interaction between Ryzodeg 100IU/ml Flextouch Pen 3 ml and nalidixic but can be taken if prescribed by a doctor. However, consult the doctor immediately if you experience hypoglycemia or hyperglycemia. Symptoms of hypoglycemia include headache, dizziness, drowsiness, nervousness, confusion, shaking, nausea, hunger, weakness, sweating, palpitation, and rapid heartbeat. Symptoms of hyperglycemia may include increased thirst, increased hunger, and increased urination. Do not stop using any medications without first talking to your doctor.
Taking Ryzodeg 100IU/ml Flextouch Pen 3 ml with sparfloxacin affects blood glucose levels, which may cause hyperglycemia (high blood glucose) and hypoglycemia less frequently.
How to manage the interaction:
There could be a possible interaction between Ryzodeg 100IU/ml Flextouch Pen 3 ml and sparfloxacin but can be taken if prescribed by a doctor. However, consult the doctor immediately if you experience hypoglycemia or hyperglycemia. Symptoms of hypoglycemia include headache, dizziness, drowsiness, nervousness, confusion, shaking, nausea, hunger, weakness, sweating, palpitation, and rapid heartbeat. Symptoms of hyperglycemia may include increased thirst, increased hunger, and increased urination. Do not stop using any medications without talking to a doctor.
Taking Ryzodeg 100IU/ml Flextouch Pen 3 ml with Lomefloxacin affects blood glucose levels, which may cause hyperglycemia (high blood glucose) and hypoglycemia (low blood glucose) less frequently.
How to manage the interaction:
There could be a possible interaction between Ryzodeg 100IU/ml Flextouch Pen 3 ml and Lomefloxacin but can be taken if prescribed by a doctor. However, consult the doctor immediately if you experience hypoglycemia or hyperglycemia. Symptoms of hypoglycemia include headache, dizziness, drowsiness, nervousness, confusion, shaking, nausea, hunger, weakness, perspiration, palpitation, and rapid heartbeat. Symptoms of hyperglycemia may include increased thirst, increased hunger, and increased urination. Do not stop using any medications without first talking to your doctor.
Taking Ryzodeg 100IU/ml Flextouch Pen 3 ml with Enoxacin affects blood glucose levels, which may cause hyperglycemia (high blood glucose) and hypoglycemia(low blood glucose) less frequently.
How to manage the interaction:
There could be a possible interaction between Ryzodeg 100IU/ml Flextouch Pen 3 ml and cinoxacin but can be taken if prescribed by a doctor. However, consult the doctor immediately if you experience hypoglycemia or hyperglycemia. Symptoms of hypoglycemia include headache, dizziness, drowsiness, nervousness, confusion, tremor, nausea, hunger, weakness, perspiration, palpitation, and rapid heartbeat. Symptoms of hyperglycemia may include increased thirst, increased hunger, and increased urination. Do not stop using any medications without first talking to your doctor.
Taking Ryzodeg 100IU/ml Flextouch Pen 3 ml with ciprofloxacin can effect blood sugar levels, both hyperglycemia (high blood sugar) and, less frequently, hypoglycemia (low blood sugar).
How to manage the interaction:
There could be a possible interaction between Ryzodeg 100IU/ml Flextouch Pen 3 ml and Ciprofloxacin but can be taken if prescribed by a doctor. However, consult the doctor immediately if you experience headache, dizziness, drowsiness, nervousness, confusion, tremor, nausea, hunger, weakness, excessive sweating, and rapid heartbeat, increased thirst, increased hunger, and increased urination. Do not stop using any medications without talking to a doctor.
Taking Ryzodeg 100IU/ml Flextouch Pen 3 ml with Gemifloxacin affects blood glucose levels, which may cause hyperglycaemia (high blood glucose) and hypoglycaemia (low blood glucose) less frequently.
How to manage the interaction:
There could be a possible interaction between Ryzodeg 100IU/ml Flextouch Pen 3 ml and Gemifloxacin but can be taken if prescribed by a doctor. However, consult the doctor immediately if you experience hypoglycaemia or hyperglycaemia. Symptoms of hypoglycaemia include headache, dizziness, drowsiness, nervousness, confusion, tremor, nausea, hunger, weakness, sweating, palpitation, and rapid heartbeat. Symptoms of hyperglycaemia may include increased thirst, increased hunger, and increased urination. Do not stop using any medications without first talking to your doctor.
Taking Ryzodeg 100IU/ml Flextouch Pen 3 ml with Moxifloxacin affects blood glucose levels, which may cause hyperglycemia (high blood glucose) and hypoglycemia (low blood glucose) less frequently.
How to manage the interaction:
There could be a possible interaction between Ryzodeg 100IU/ml Flextouch Pen 3 ml and Moxifloxacin but can be taken if prescribed by a doctor. However, consult the doctor immediately if you experience headache, dizziness, and rapid heartbeat. Symptoms of hyperglycemia may include increased thirst, and increased urination. Do not stop using any medications without talking to a doctor.
Drug-Food Interactions
Drug-Food Interactions
Login/Sign Up
Drug-Diseases Interactions
Drug-Diseases Interactions
Login/Sign Up
Drug-Drug Interactions Checker List
- ASPIRIN
- ISONIAZID
- PENTAMIDINE
- CIPROFLOXACIN
- GLUCAGON
- DANAZOL
- METFORMIN
- RESERPINE
- NIACIN
- ATENOLOL
- ACEBUTOLOL
- METOPROLOL
- LEVOTHYROXINE
- FLUOXETINE
- LITHIUM
- PROGESTERONE
- ESTROGEN
Habit Forming
Special Advise
- Your doctor may tell you to have a regular potassium level as prolonged administration causes a life-threatening hypokalemia condition (low potassium).
- Store Ryzodeg 100IU/ml Flextouch Pen 3 ml between 2-8 degree celsius temperature only. Do not store Ryzodeg 100IU/ml Flextouch Pen 3 ml in the freezer.
Diet & Lifestyle Advise
- Exercise may lower your body’s need for insulin during and for some time after the physical activity.
- Exercise may also speed up an insulin dose effect, especially if the exercise involves the injection site area (for example, the leg should not be used for injection just before running).
- Discuss with your doctor how you should adjust your insulin regimen to accommodate exercise.
- Avoid eating sugar food and prefer food cooked food low in calories.
- When travelling across more than 2 time zones, you should talk to your doctor about your insulin schedule adjustments.
All Substitutes & Brand Comparisons
RX
Ryzodeg 100IU/ml Penfill 3 ml
Abbott India Ltd
₹1238.5
(₹412.83/ 1ml)
22% CHEAPER

Have a query?
Buy best Diabetics products by
Torrent Pharmaceuticals Ltd
Sun Pharmaceutical Industries Ltd
Eris Life Sciences Ltd
Intas Pharmaceuticals Ltd
Lupin Ltd
Micro Labs Ltd
Mankind Pharma Pvt Ltd
Lloyd Healthcare Pvt Ltd
Alkem Laboratories Ltd
Abbott India Ltd
Glenmark Pharmaceuticals Ltd
Cipla Ltd
Macleods Pharmaceuticals Ltd
Wockhardt Ltd
Dr Reddy's Laboratories Ltd
Primus Remedies Pvt Ltd
USV Pvt Ltd
Aristo Pharmaceuticals Pvt Ltd
Emcure Pharmaceuticals Ltd
Alembic Pharmaceuticals Ltd
Ipca Laboratories Ltd
La Renon Healthcare Pvt Ltd
Ajanta Pharma Ltd
Medley Pharmaceuticals Ltd
East West Pharma India Pvt Ltd
Elbrit Life Sciences Pvt Ltd
Corona Remedies Pvt Ltd
Hbc Life Sciences Pvt Ltd
Sinsan Pharmaceuticals Pvt Ltd
Ranmarc Labs
Mitoch Pharma Pvt Ltd
Zydus Healthcare Ltd
Sanofi India Ltd
Akumentis Healthcare Ltd
Fusion Health Care Pvt Ltd
Unison Pharmaceuticals Pvt Ltd
Jubilant Lifesciences Ltd
Novo Nordisk India Pvt Ltd
Tas Med India Pvt Ltd
Blue Cross Laboratories Pvt Ltd
Msn Laboratories Pvt Ltd
Eswar Therapeutics Pvt Ltd
Indoco Remedies Ltd
Q Check Pharmaceuticals
Alteus Biogenics Pvt Ltd
Anthem Bio Pharma
Franco Indian Pharmaceuticals Pvt Ltd
Systopic Laboratories Pvt Ltd
Panacea Biotec Ltd
Zydus Cadila
Biocon Ltd
Edoc Life Sciences Pvt Ltd
Koye Pharmaceuticals Pvt Ltd
Arkas Pharma Pvt Ltd
Diacardus Pharmacy Pvt Ltd
Elinor Pharmaceuticals (P) Ltd
Remedy Life Sciences Pvt Ltd
Saan Labs
Talent India Pvt Ltd
Jarun Pharmaceuticals Pvt Ltd
Capital Pharma
Shrrishti Health Care Products Pvt Ltd
FDC Ltd
Leeford Healthcare Ltd
Nirvana India Pvt Ltd
Elder Pharmaceuticals Ltd
Eli Lilly and Company (India) Pvt Ltd
Glynis Pharmaceuticals Pvt Ltd
Zuventus Healthcare Ltd
Arrient Healthcare Pvt Ltd
Cadomed Pharmaceuticals India Pvt Ltd
Orris Pharmaceuticals
Akesiss Pharma Pvt Ltd
Bal Pharma Ltd
Biochem Pharmaceutical Industries Ltd
Knoll Healthcare Pvt Ltd
Lippon Pharma Pvt Ltd
Morepen Laboratories Ltd
Neucure Lifesciences Pvt Ltd
Opsis Care Lifesciences Pvt Ltd
Wallace Pharmaceuticals Pvt Ltd
Acmedix Pharma Llp
Converge Biotech Pvt Ltd
Erinyle Pharma
Indiabulls Pharmaceuticals Pvt Ltd
Ozone Pharmaceuticals Ltd
Retra Life Science Pvt Ltd
Alvio Pharmaceuticals Pvt Ltd
Geneaid Pharmaceuticals
Heal (India) Laboratories Pvt Ltd
Olcare Laboratories Pvt Ltd
Vasu Organics Pvt Ltd
Kotak Life Sciences
Lakshya Life Sciences Pvt Ltd
Proqol Health Care Pvt Ltd
Sanz Pharmaceuticals
Daylon healthcare pvt Ltd
Mcronus Lifescience Pvt Ltd
Natco Pharma Ltd
Orsim Pharma
Customers Also Bought