Okavax Vaccine

MRP ₹1260
(Inclusive of all Taxes)
₹189.0 Cashback (15%)
know your delivery time
Provide Delivery Location
Composition :
Manufacturer/Marketer :
Consume Type :
Expires on or after :
Return Policy :

Secure Payment

Trusted by 8 Crore Indians

Genuine Products
Therapeutic Class
Country of origin
Manufacturer/Marketer address
Disclaimer
Alcohol
Safe if prescribed
Do not consume alcohol with Okavax Vaccine as it may result in serious side effects.
Pregnancy
Consult your doctor
Okavax Vaccine is not recommended during pregnancy. If you are pregnant, inform your doctor before taking this medicine.
Breast Feeding
Consult your doctor
Okavax Vaccine is probably safe to use while breastfeeding. According to limited human data, the drug poses no significant risk to the baby.
Driving
Safe if prescribed
Okavax Vaccine may not affect your ability to drive.
Liver
Consult your doctor
Okavax Vaccine does not affect liver function. However, if you have liver problems, your doctor will weigh the benefits and risks before prescribing this medication based on your condition.
Kidney
Consult your doctor
Okavax Vaccine does not affect kidney function. However, if you have kidney problems, your doctor will weigh the benefits and risks before prescribing this medication based on your condition.
Children
Safe if prescribed
Okavax Vaccine should not be given to children under nine months of age. In children from 9 months to 12 years of age, Okavax Vaccine should be used with caution as dosage adjustments are necessary.
Keep Refrigerated. Do not freeze.Prepaid payment required.
About Okavax Vaccine
Okavax Vaccine belongs to the class of medications called ‘immunizing agents’ used to prevent varicella or chickenpox. Chickenpox is a vaccine-preventable viral infection caused by the varicella-zoster virus. It is highly contagious, especially in people who have never had the disease or have not been vaccinated against it. Symptoms include rash, small painful blisters, fever, sore throat, and body pain.
Okavax Vaccine is an immunizing agent or vaccine made from a live, weakened or attenuated varicella-zoster virus. It helps to provide persistent or long-lasting protection against varicella or chickenpox. Okavax Vaccine helps develop immunity by forming antibodies by stimulating the immune system. It is essential to take the vaccine doses per the doctor’s advice for effective protection against the disease.
Okavax Vaccine will be administered by a healthcare professional; hence do not self-administer. Okavax Vaccine may cause side effects such as fever, pain, swelling, and redness at the injection site. These side effects are mild and temporary. However, if these side effects persist or worsen, inform your doctor immediately.
It is not recommended to take Okavax Vaccine if you are allergic to neomycin or any contents present in this medicine. Okavax Vaccine should not be given to people with blood disorders or cancer of the blood (leukaemia) or immune system (lymphoma), with a weak immune system, such as patients with HIV or AIDS, who are receiving immunosuppressants or receiving high doses of corticosteroids, who have active untreated tuberculosis, and who have a temperature higher than 38.50c Okavax Vaccine should not be given to pregnant women and used with caution in breastfeeding mothers. Okavax Vaccine is not recommended for children below 9 months of age. Okavax Vaccine should be used with caution in elderly people. Okavax Vaccine may not interact with alcohol and may not affect your driving ability.
Uses of Okavax Vaccine
Medicinal Benefits Mweb
Key Benefits
Okavax Vaccine provides active immunization against varicella-zoster virus or chickenpox disease for healthy individuals above nine months of age. It benefits people who are non-immune and at risk of infection, such as healthcare workers or workers in children's day-care centres, non-immune parents of young children, and non-immune household contacts of patients with weak immune systems with no history of the disease. Vaccination within three days of exposure to the virus can reduce the risk of infection and the severity of the disease.
Directions for Use
Side Effects of Okavax Vaccine
- Fever
- Pain or redness at the injection site
- Swelling at the injection site
Drug Warnings
People who have been vaccinated with Okavax Vaccine should avoid close contact for up to 6 weeks with the following individuals: who have not been previously vaccinated against chickenpox, children/adults/pregnant women who have never had chickenpox, who have a weak immune system or receiving immune suppressants, and new-born babies whose mother have never had chickenpox. Adults and children with weak immune systems who receive Okavax Vaccine should be closely monitored as this vaccine is less effective in these people. In people who have undergone blood or plasma transfusion, who had taken human normal immunoglobulin or varicella-zoster immunoglobulin, Okavax Vaccine vaccine should be avoided or postponed for at least five months in these people. Besides, people should not receive any immunoglobulin medicines for one month following Okavax Vaccine . You should not take pain killer (aspirin) for six weeks following Okavax Vaccine as it may cause Reye syndrome (a severe condition that causes liver and brain damage). Women of childbearing potential should use reliable contraceptive methods as it is not recommended to get pregnant for one month following Okavax Vaccine vaccination.
Drug-Drug Interactions Checker List
- TACROLIMUS
- CYCLOSPORINE
- AZATHIOPRINE
- PREDNISONE
- METHYLPREDNISOLONE
- DEXAMETHASONE
- ASPIRIN
- HUMAN NORMAL IMMUNOGLOBULIN
- VARICELLA ZOSTER IMMUNE GLOBULIN
Habit Forming
Special Advise
- Okavax Vaccine should not be given intravenously (IV). It is given under the skin or into the muscle. If given into the muscle, the upper arm area is preferred in adults, and the thigh area is preferred in children. The injection is usually given under the skin if you have a blood clotting disorder or thrombocytopenia (low levels of platelets).
- If there is a requirement to give more than one live virus vaccine simultaneously, these injections should be given at different sites. However, if it is not necessary to give them on the same day, they should be spaced at an interval of at least four weeks.
Diet & Lifestyle Advise
- Eat bland foods such as rice, pasta, and oatmeal and soft foods such as mashed potatoes, sweet potatoes, boiled eggs, avocado, and boiled chicken.
- Prefer eating yoghurt, milkshakes, and smoothies. Avoid eating spicy, salty, and acidic foods.
- Stay hydrated. Prefer drinks such as coconut water, herbal teas, low-sugar drinks, and electrolyte-infused drinks.
- Do not scratch or itch the rashes, as it may worsen them. Regular hand washing and trimming the nails can avoid the spreading of infection.
- Monitor your temperature regularly. Sponging can help to reduce fever.
- Take adequate rest and sleep.
- Stay isolated and do not go outside until the last blister has disappeared.
- Vaccinate all the contacts (family members or caretakers) within 3 to 5 days post-exposure to the virus.
All Substitutes & Brand Comparisons
RX
Out of StockNexipox Vaccine 1's
Novo Medi Sciences Pvt Ltd
₹2427
(₹2184.3 per unit)
3% CHEAPERRX
Out of StockZuvicella 0.5Ml Vaccine
Zuventus Healthcare Ltd
₹1740
(₹3132.0/ 1ml)
38% COSTLIERRX
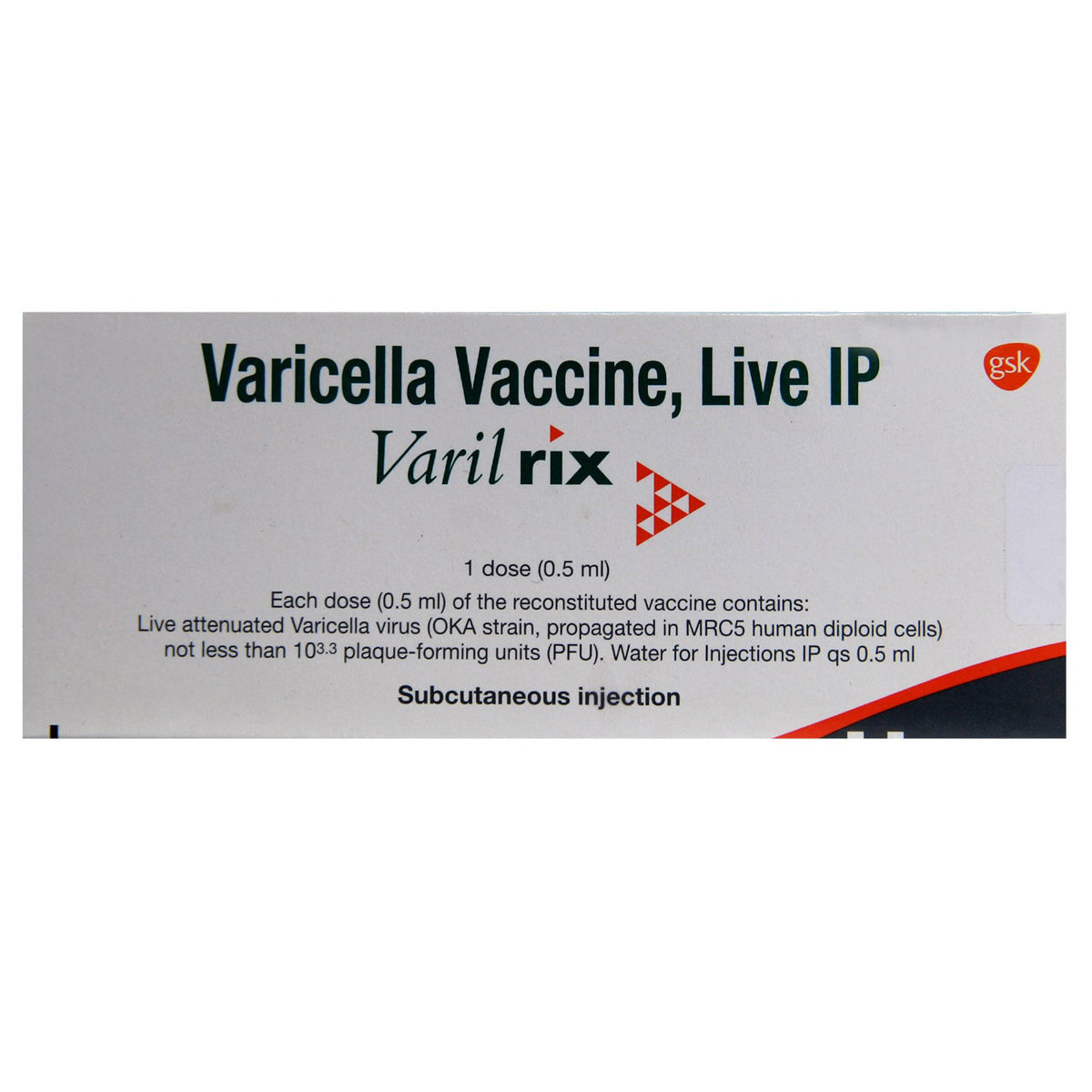
Varilrix Vaccine 0.5 ml
GlaxoSmithKline Pharmaceuticals Ltd
₹2418
(₹4255.6/ 1ml)
87% COSTLIER

Have a query?
Buy best Vaccines products by
Serum Institute Of India Pvt Ltd
GlaxoSmithKline Pharmaceuticals Ltd
Biological E Ltd
Abbott India Ltd
Bharat Biotech
Sanofi India Ltd
Human Biologicals Institute
Msd Pharmaceutical Pvt Ltd
Panacea Biotec Ltd
Zydus Healthcare Ltd
Bharat Sanchar Nigam Ltd
Cadila Healthcare Ltd
Lupin Ltd
Zuventus Healthcare Ltd
Bharat Serums and Vaccines Ltd
Biomed
Indian Immunologiclas Ltd
Novartis India Ltd
Pfizer Ltd
Baxter India Pvt Ltd
Bharath
Bio-Med Pvt Ltd
Biomed Pharma
Cpl Biologicals Pvt Ltd
Dr Reddy's Laboratories Ltd
Indiabulls Pharmaceuticals Pvt Ltd
Indian Drugs & Pharmaceuticals Ltd
Kamada Pharmaceuticals
Mankind Pharma Pvt Ltd
Novamed Pharma
Novo Medi Sciences Pvt Ltd
Ranbaxy Laboratories Ltd
Samarth Life Sciences Pvt Ltd
Shantha Biotech
Smith & Kenner Pharma Pvt Ltd
Synergy Pharmaceuticals
Unison Pharmaceuticals Pvt Ltd
Vins Bio Products Ltd
Wockhardt Ltd
Zydus Cadila