Myfortic 360 mg Tablet

MRP ₹1141
(Inclusive of all Taxes)
₹57.0 Cashback (5%)
know your delivery time
Provide Delivery Location
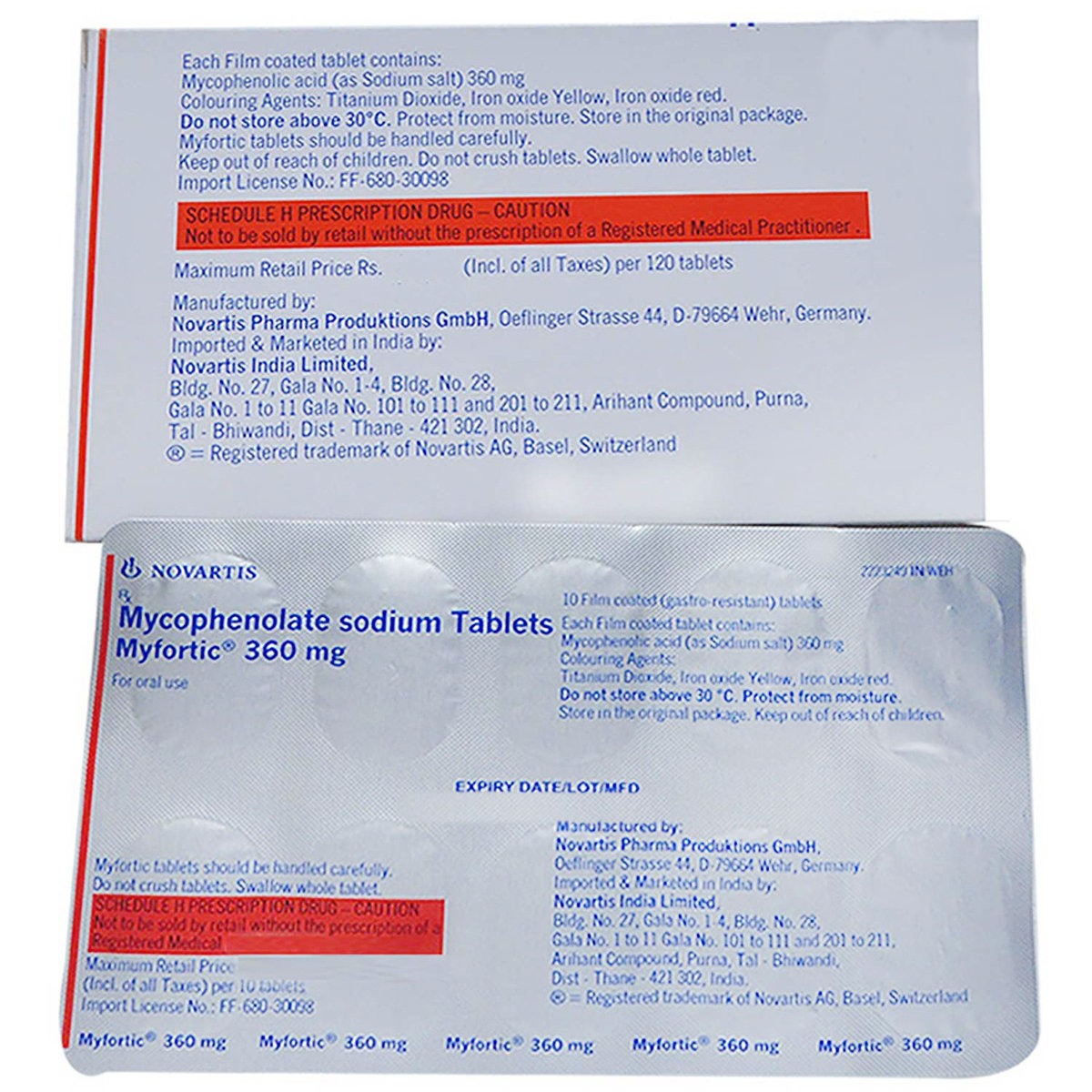
Composition :
Manufacturer/Marketer :
Consume Type :
Expires on or after :
Return Policy :

Secure Payment

Trusted by 8 Crore Indians

Genuine Products
Therapeutic Class
Country of origin
Manufacturer/Marketer address
FAQs
Disclaimer
Alcohol
Safe if prescribed
You are recommended to avoid alcohol consumption while taking Myfortic 360 mg Tablet . It could lead to increased dizziness and drowsiness.
Pregnancy
Consult your doctor
Myfortic 360 mg Tablet may cause birth defects and miscarriage if taken during pregnancy. Follow contraception advice given by your doctor if you are a woman who could become pregnant. Also, undergo a pregnancy test before starting treatment with Myfortic 360 mg Tablet .
Breast Feeding
Consult your doctor
It is not recommended to take Myfortic 360 mg Tablet while you are breastfeeding. Myfortic 360 mg Tablet passes into breast milk.
Driving
Safe if prescribed
Myfortic 360 mg Tablet may cause dizziness, tiredness, do not drive or operate heavy machinery if you feel dizzy.
Liver
Consult your doctor
Take Myfortic 360 mg Tablet only after the doctor's prescription, especially if you have liver diseases/conditions as your doctor may adjust the dose as required.
Kidney
Consult your doctor
Please consult your doctor if you have kidney impairment before taking Myfortic 360 mg Tablet as your doctor may adjust the dose as required.
Children
Safe if prescribed
Myfortic 360 mg Tablet should not be used by children below 18 years of age, as the efficacy and safety have not been established.
Product Substitutes
About Myfortic 360 mg Tablet
Myfortic 360 mg Tablet belongs to a class of medications called ‘immunosuppressants’ used to prevent the body from rejecting a transplanted organ such as a kidney, heart or liver. Transplant rejection occurs when the recipient’s immune system identifies the transplanted organ as a ‘foreign object’ and attacks it. If it is not treated promptly, it may cause irreversible damage.
Myfortic 360 mg Tablet contains ‘Mycophenolate sodium’ used in the treatment of organ transplant rejection along with another immunosuppressant and anti-inflammatory agent. It inhibits T and B lymphocytes (white blood cells that attack foreign cells) and suppresses antibodies' production (identify and kill foreign cells). These effects suppress the immune system so that the transplanted graft is not rejected.
You are advised to take Myfortic 360 mg Tablet for as long as your doctor has prescribed it for you depending on your medical condition. In some cases, you may experience certain common side-effects, such as diarrhoea, cough, muscle pain, low blood pressure, fever and respiratory infections. Most of these side-effects do not require medical attention and will resolve gradually over time. However, you are advised to talk to your doctor if you experience these side-effects persistently.
To treat your condition effectually, continue taking Myfortic 360 mg Tablet for as long as your doctor has prescribed. To avoid recurring symptoms, do not stop it midway. Do not take Myfortic 360 mg Tablet if you are pregnant or planning for pregnancy. Myfortic 360 mg Tablet should not be taken by breastfeeding mothers as it passes into breast milk. Myfortic 360 mg Tablet should not be given to children as safety and efficacy have not been established. Myfortic 360 mg Tablet may make you more susceptible to infections, consult your doctor if you develop fever, sore throat, breathlessness, jaundice, unexplained bleeding or bruising. Avoid excessive exposure to sunlight, wear protective clothing, and use protective sunscreen with a high protection factor while using Myfortic 360 mg Tablet . Do not contact anyone who has shingles, chickenpox, or measles. Do not take Myfortic 360 mg Tablet if you are not using effective contraception.
Uses of Myfortic 360 mg Tablet
Medicinal Benefits Mweb
Key Benefits
Myfortic 360 mg Tablet contains ‘Mycophenolate sodium’ which belongs to the class of ‘immunosuppressants’. It is used to prevent organ transplant rejection. It reduces the activity of the immune system by inhibiting the action of white blood cells (responsible for immune reactions) in the body.
Directions for Use
Side Effects of Myfortic 360 mg Tablet
- Dizziness
- Headache
- Cough
- Respiratory infections
- Weakness (asthenia)
- Muscle pain (myalgia)
- Shortness of breath (dyspnoea)
Drug Warnings
Do not take Myfortic 360 mg Tablet if you are allergic to any immunosuppressant medicines or mycophenolate sodium. Let your doctor know before using Myfortic 360 mg Tablet if you have liver or kidney problems, severe digestive issues, cancer, liver disease (such as hepatitis B, hepatitis C), current/past infections, rare genetic disorders (such as Lesch-Nyhan or Kelley-Seegmiller syndromes). Do not take Myfortic 360 mg Tablet if you are pregnant or planning for pregnancy as Myfortic 360 mg Tablet is a Pregnancy category risk D medicine which can cause serious congenital disabilities to the unborn baby. Myfortic 360 mg Tablet should not be taken by breastfeeding mothers as it passes into breast milk. Pregnant women should use reliable forms of birth control while on treatment and for six weeks after stopping treatment with Myfortic 360 mg Tablet . While taking Myfortic 360 mg Tablet , do not contact anyone who has shingles, chickenpox, or measles. Myfortic 360 mg Tablet causes dizziness and drowsiness, so drive with caution. Myfortic 360 mg Tablet should not be given to children as safety and efficacy have not been established. Avoid consuming alcohol along with Myfortic 360 mg Tablet as it could lead to increased dizziness. Your doctor may advise you to undergo regular blood tests, kidney and liver tests to monitor your condition. Avoid excessive exposure to sunlight, wear protective clothing, and use protective sunscreen with a high protection factor while using Myfortic 360 mg Tablet .
Drug-Drug Interactions
Drug-Drug Interactions
Login/Sign Up
Coadministration of Colestipol with Myfortic 360 mg Tablet can reduce its absorption and action in the body. This can lead to low treatment results.
How to manage the interaction:
Taking Myfortic 360 mg Tablet with Colestipol is not recommended as it can result in an interaction, it can be taken if your doctor has advised it.
Coadministration of Myfortic 360 mg Tablet and Tofacitinib can increase the risk or severity of developing serious infections
How to manage the interaction:
Taking Myfortic 360 mg Tablet and Tofacitinib together can possibly result in an interaction, it can be taken if your doctor has advised it. However, if you experience fever, chills, diarrhoea, sore throat, muscle aches, shortness of breath, blood in phlegm, weight loss, red or inflamed skin, body sores, and pain or burning during urination consult a doctor. Do not discontinue any medications without a doctor's advice.
Coadministration of Myfortic 360 mg Tablet and Desogestrel can decrease the levels and action of Desogestrel.
How to manage the interaction:
Co-administration of Desogestrel with Myfortic 360 mg Tablet can possibly result in an interaction, but it can be taken if your doctor has advised it. If you notice any of these symptoms: abnormal menstruation, dryness of the vagina, or increased hot flushes or if you're looking for other birth control options, consult a doctor. Do not discontinue any medications without consulting a doctor.
Coadministration of Ethinylestradiol with Myfortic 360 mg Tablet may lower the blood levels and effects of Ethinylestradiol, making it less effective as a form of birth control.
How to manage the interaction:
There could be a possible interaction between Myfortic 360 mg Tablet and Ethinylestradiol, but they can be taken together if a doctor has prescribed them. However, if you are taking hormone replacement therapy for menopause, and you notice an increase in the frequency or severity of your symptoms, such as hot flashes, vaginal dryness, or irregular bleeding, consult a doctor. Do not discontinue any medications without a doctor's advice.
Coadministration of Norethisterone and Myfortic 360 mg Tablet can decrease the levels and effects of Norethisterone.
How to manage the interaction:
There could be a possible interaction between Myfortic 360 mg Tablet and Norethisterone, but they can be taken together if your doctor has prescribed them. However, if you experience hot flashes, vaginal dryness, or abnormal bleeding consult a doctor. Do not discontinue any medications without a doctor's advice.
A combined use of Levonorgestrel with Myfortic 360 mg Tablet may reduce the blood levels and effects of levonorgestrel, which may make it less reliable as a form of birth control, Myfortic 360 mg Tablet may cause pregnancy loss in the first trimester and also birth defects in the unborn child.
How to manage the interaction:
There could be a possible interaction between Myfortic 360 mg Tablet and Levonorgestrel, but they can be taken together if a doctor has prescribed them. However, if you take hormone replacement (Levonorgestrel) for menopause, and if you experience hot flashes, vaginal dryness, or abnormal bleeding consult a doctor. Do not discontinue any medications without a doctor's advice.
Coadministration of Myfortic 360 mg Tablet and Colestyramine can decrease the effect of Myfortic 360 mg Tablet.
How to manage the interaction:
There could be a possible interaction between Myfortic 360 mg Tablet and Cholestyramine, it can be taken together if your doctor has prescribed them. However, if you experience fever, diarrhea, sore throat, muscle aches, shortness of breath, inflamed skin, body pain, or burning during urination consult a doctor. Do not discontinue any medications without a doctor's advice.
Taking Myfortic 360 mg Tablet and Golimumab can lead to or increase the risk or severity of developing serious infections.
How to manage the interaction:
There could be a possible interaction between Myfortic 360 mg Tablet and Golimumab, but they can be taken together if your doctor has prescribed them. However, if you experience fever, chills, diarrhoea, sore throat, muscle aches, shortness of breath, blood in phlegm, weight loss, red or inflamed skin, body sores, and pain or burning during urination consult a doctor. Do not discontinue any medications without a doctor's advice.
Coadministration of medroxyprogesterone with Myfortic 360 mg Tablet may decrease the levels and effects of medroxyprogesterone.
How to manage the interaction:
There could be a possible interaction between Myfortic 360 mg Tablet and Medroxyprogesterone, but they can be taken together if your doctor has prescribed them. However, if you experience hot flashes, vaginal dryness, or abnormal bleeding consult a doctor. Do not discontinue any medications without a doctor's advice.
Co-administration of Ferrous sulfate with Myfortic 360 mg Tablet decreases levels of Mycophenolate by reducing its absorption.
How to manage the interaction:
There could be a possible interaction between Myfortic 360 mg Tablet and Ferrous sulfate, but they can be taken together if a doctor has prescribed them. However, if you experience upset stomach, nausea, vomiting, diarrhea, constipation sleep problems, fatigue, headache, painful urination, high blood pressure, or swelling of the lower legs, ankles, or feet, joint pain, cough, or shortness of breath consult a doctor. Do not discontinue any medications without a doctor's advice.
Drug-Food Interactions
Drug-Food Interactions
Login/Sign Up
Drug-Diseases Interactions
Drug-Diseases Interactions
Login/Sign Up
Drug-Drug Interactions Checker List
- TACROLIMUS
- CHOLESTYRAMINE
- ACTIVATED CHARCOAL
- ACICLOVIR
- GANCICLOVIR
Habit Forming
Special Advise
- Use sunscreen while taking Myfortic 360 mg Tablet , as it can make your skin more sensitive to sunlight and may cause skin cancer.
- Do not donate blood while using this medication and for 6 weeks after stopping it. Do not donate sperm while using this medication and for 90 days after stopping it.
Diet & Lifestyle Advise
- Physical activity helps in strengthening muscles and relieves joint stiffness. Gentle activities like 20-30minutes of walking or swimming would be helpful.
- Performing yoga may also help in improving joint flexibility and pain management.
- Maintain a healthy weight by performing regular low-strain exercises and eating healthy food.
- Get adequate sleep as resting the muscles can help in reducing inflammation and swelling.
- Follow heat or cold therapy, apply a cold or hot compress on the joints for 15-20minutes regularly.
- De-stress yourself by meditating, reading books, taking a warm bubble bath or listen to soothing music.
- Acupuncture, massage and physical therapy may also be helpful.
- Eat food rich in antioxidants such as berries, spinach, kidney beans, dark chocolate, etc.
- Foods containing flavonoids help in reducing inflammation. These include soy, berries, broccoli, grapes, and green tea.
All Substitutes & Brand Comparisons
RX
My 360 Tablet 10's
Steadfast MediShield Pvt Ltd
₹600
(₹54.0 per unit)
52% CHEAPERRX
MMF-S Tablet 10's
Ipca Laboratories Ltd
₹659.5
(₹59.36 per unit)
47% CHEAPERRX
Ipca MMF-S Tablet 10's
Ipca Laboratories Ltd
₹659.5
(₹59.36 per unit)
47% CHEAPER

Have a query?
Buy best Immuno Modulators products by
Intas Pharmaceuticals Ltd
Ipca Laboratories Ltd
Panacea Biotec Ltd
Cipla Ltd
Emcure Pharmaceuticals Ltd
Biocon Ltd
Hetero Healthcare Pvt Ltd
RPG Life Sciences Ltd
Reliance Formulation Pvt Ltd
Sun Pharmaceutical Industries Ltd
Bharat Serums and Vaccines Ltd
Novartis India Ltd
Zydus Healthcare Ltd
Overseas Health Care Pvt Ltd
Prevego Healthcare & Research Pvt Ltd
Wallace Pharmaceuticals Pvt Ltd
Zydus Cadila
Alkem Laboratories Ltd
Alembic Pharmaceuticals Ltd
Cadila Healthcare Ltd
Knoll Healthcare Pvt Ltd
La Renon Healthcare Pvt Ltd
Msn Laboratories Pvt Ltd
Abbott India Ltd
Concord Biotech Ltd
EVERVITAL LIFESCIENCES
Mylan Pharmaceuticals Pvt Ltd
Steadfast MediShield Pvt Ltd
Tas Med India Pvt Ltd
Wockhardt Ltd
Anthem Bio Pharma
Hetero Drugs Ltd
Hospimax Healthcare Pvt Ltd
Indiabulls Pharmaceuticals Pvt Ltd
Micro Labs Ltd
Biotest Pharma Gmbh
Brinton Pharmaceuticals Ltd
CONCORD DRUGS LTD
Calren Care Lifesciences Pvt Ltd
Celon Laboratories Pvt Ltd
Cognitus Life Sciences Pvt Ltd
Medgenix Pharma India Pvt Ltd
Mediart Life Sciences Pvt Ltd
Natco Pharma Ltd
Rene Lifescience
Vasu Organics Pvt Ltd
Alniche Life Sciences Pvt Ltd
Ankaa Pharmaceutical
Arcalis India Pharmaceuticals Pvt Ltd
Astellas Pharma India Pvt Ltd
Bharat Sanchar Nigam Ltd
Canixa Life Sciences Pvt Ltd
Care Formulations Lab
Celera Healthcare Pvt Ltd
Concord Laboratories Pvt Ltd
Dr Reddy's Laboratories Ltd
Immune Biotech Pvt Ltd
Lupin Ltd
Oxygen Pharma Care Pvt Ltd
Plasmagen Biosciences Pvt Ltd
Rhumasafe Pharma
Rivan Pharmaceuticals Pvt Ltd
Rockmed Pharma Pvt Ltd
The Madras Pharmaceuticals
Ajanta Pharma Ltd
Akognos Life Science Pvt Ltd
Aubade Healthcare Pvt Ltd
Biogen Idec Biotech India Pvt Ltd
Cadila Pharmaceuticals Ltd
Celera Pharma Pvt Ltd
East West Pharma India Pvt Ltd
Elera Pharma Ltd
Eli Lilly and Company (India) Pvt Ltd
Enactis Healthcare Pvt Ltd
Entero Healthcare Solution Pvt Ltd
Fresenius Kabi India Pvt Ltd
Galcare Pharmaceuticals Pvt Ltd
Getwell Oncology Pvt Ltd
Glasier Wellness Inc
GlaxoSmithKline Pharmaceuticals Ltd
Glenmark Pharmaceuticals Ltd
J B Chemicals & Pharmaceuticals Ltd
Neuten HealthCare
Pfizer Ltd
Renauxe Pharma India Pvt Ltd
Sai Mirra Innopharma Pvt Ltd
Steris Healthcare
Torrent Pharmaceuticals Ltd
United Biotech Pvt Ltd
Virchow Biotech Pvt Ltd
Actus Health Care
Alacris Healthcare Pvt Ltd
Ant Pharmaceuticals Pvt Ltd
Assentus Biogenics Pvt Ltd
Aureate Healthcare
Azista Industries Pvt Ltd
BDH Industries Ltd
BSA Pharma Inc
Bharat Biotech
Bioceutics Inc

Frequently Bought Together
Recommended for a 30-day course: 4 Strips